SHUCHI.P.NAHAR
The Indian pharmaceutical companies always had strong chemistry skills to work on small molecules to make generic drugs, but they lacked skills, people and ecosystem to work on large molecules to come up with reverse engineered versions of biotech drugs, many experts complain. However, it is beginning to change.
'India: the emerging hub for biologics and biosimilars', the Indian pharmaceutical companies have launched 123 biosimilars and currently have 201 active biosimilars covering multiple indications in the development pipeline.
Further, India has 95 approved biosimilars in the domestic market, more than any other country in the world.
Biologics are the biological products regulated under the rules of the Food and Drug Administration (FDA). They are used to either diagnose, prevent, treat, or cure various medical conditions.
Significantly, biologics are large, complex, and diverse molecules produced through biotechnology. And, this uses living systems including animal cells, plant cells or microorganisms. Some biologics are therapeutic proteins such as filgrastim, monoclonal antibodies such as adalimumab,vaccines such as for influenza and tetanus, etc.
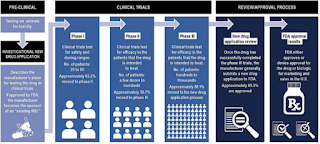
Moreover, the main challenge of manufacturing biologics is the consequent inherent variations These variations of products have to be characterized with time. Therefore, the FDA assesses the manufacturer's strategy as well as the manufacturing procedure in order to control the product variations.
The biologic market is set to grow, with biologic drug sales forecast to increase from $239bn in 2018 to $493bn by 2025, representing a compound annual growth rate of 11%, according to GlobalData’s Drug Sales and Consensus Forecast (DSCF) Database.
Furthermore, with biologics making up 41% of pipeline drugs, combined with investor understanding of these drugs’ potential and ongoing attractive IPO conditions across all markets, we can expect to see further biologic IPOs in the very near future.
Biologics are one of the top selling drugs worldwide as well as in the United States but the major
drawback of this drug has been its exorbitant cost,which makes it unaffordable and inaccessible to many patients, especially in developing countries where a large number of people are poor and the concept of health insurance is at its nascent stage.
But the silver lining is that once the innovator company loses their intellectual property right and patent protection after a stipulated period, it opens the window of opportunities for companies evince an interest in manufacturing similar products, which cost less, and at that time, it is known as biosimilar or similar biologic.
Biosimilar is a biologic product, which is very similar to Food and Drug Administration (FDA)-approved biological product known as reference product and has no clinically meaningful differences in term of safety and effectiveness from the reference product.
But similar biologics are not exactly identical to reference biologics because of the complex structure, which may be affected by minor alteration in sequences and post translational modification
Degree of Complexity in Biologics
Unlike generic medicines, manufactured through chemical synthesis, biologic medicines contain one or more active principles produced or derived from a biologic source, including a wide range of products like vaccines, allergens or recombinant proteins.

Most biologics, such as monoclonal antibodies, have a highly complex molecular structure that is difficult to identify or characterize and they are manufactured using the most innovative technologies.
In biologics, monoclonal antibodies have relevance in the treatment of pathologies that affect a large number of people: cancer, lymphoma, rheumatoid arthritis, psoriasis, ulcerative colitis.
Biosimilars are medicines manufactured through biotechnology and which have demonstrated their equivalence in quality, efficacy and safety to the reference product.
What is the Opportunity for India?
The global biologics market size was valued at USD 276.6 billion in 2015. These products represent cutting-edge research and also enable the latest scientific discoveries. Such discoveries can be translated into novel therapies that provide new treatment options for patients.
Moreover, an increased understanding of the genetic and molecular basis of disease has opened
the development of a range of targeted treatments. For instance, recombinant proteins aid the immune system in the identification and binding of foreign substances.
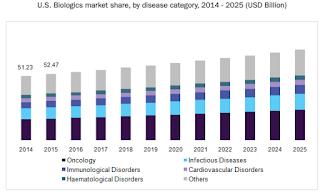
Future in Biologics
Biologics are basically products that are produced from living organisms or contain components of living organisms. They include a wide variety of products derived from humans, animals or
microorganisms using the tools of biotechnology.
Follow-on biologics and biosimilars as a class of drugs have emerged as an effective alternative
to reduce the cost of biologic therapies.
According to a report by Associated Chambers of Commerce of India (Assocham), the global market for biologics will be $240 billion and the Indian market will be over $35 bn by 2030
Indian pharma companies including Biocon, Glenmark Pharmaceuticals, and Zydus Wellness
are actively focusing on the biologics market.
Biocon, for instance, earned Rs 1,517 crore or nearly 28% revenue from biologics in FY19- FY20.
A lot will however depend upon factors such as access to cr gitical technology, regulatory guidelines and the price difference between biosimilars and the underlying biologics. For instance, the price gap between the two has significantly widened to over 60% for some drugs in Europe from just over 20% a few years ago. There is a pressing need for the biologic drugs in the global healthcare to address the unmet need in multiple therapeutic areas, particularly in oncology, nephrology, immunology, diabetes and others.
India is a leading player in the biologics market at global level. Domestic Indian companies such as: Biocon, Dr Reddy’s, Intas Pharmaceuticals, Hetero Pharma, and Lupin Pharmaceuticals are few of the key players with the largest number of products launched in the country as well as in other emerging economies.
Moreover, with growing interest in biologics in the Indian landscape, many new companies,
including startups such as Enzene Biosciences, are also jumping on the biologics bandwagon.
SHUCHI.P.NAHAR





great articles on the blog- most of them well researched, highly detailed and informative. thanks for writing and look forward to read more on your blog.
ReplyDelete