Syngene International Limited - Company Overview
Link to FY 2020-21 Annual Report takeaways - https://myweekendspot.blogspot.com/2021/10/syngene-international-ltd-fy2020-21.html
1. Company Overview
Incorporated in 1994 as a subsidiary of Biocon,
Syngene International (SIL) is a leading contract research organization (CRO),
which supports R&D programs of global innovative companies
SIL offers outsourced services to support
discovery and development for organizations across industrial sectors like
pharmaceuticals, biopharmaceuticals, nutraceuticals, animal health,
agro-chemicals, etc. It currently caters to 293 global players including
Bristol-Myers Squibb (BMS), Abbott, Baxter and Amgen, among others. SIL derives
95% of its revenues from exports. Mangalore API plant is constructed and
validation is expected to be completed in FY21. In FY20, Syngene extended its biologics discovery and preclinical
research capabilities in CAR-T therapy, an innovative cell-based approach to
treat cancer.
Received approval from the Russian Ministry of Health the approval
process was triggered as a result of a 4-year project with a Russian customer
in which Syngene supported in the development and supply of multiple,
modified-release tablet formulations of a multiple sclerosis drug.
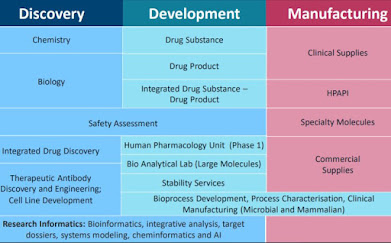
2. Integrated business model – Ground
for customer stickiness - Revenues grew at ~16% CAGR in FY16-20 to
Rs. 2012 crore due to new client addition on a regular basis and scaled up
revenues from existing clients led by integrated service offerings, high data
integrity ethos and continuous endeavour to move up the value chain.
- Eight of the top 10 global pharma companies
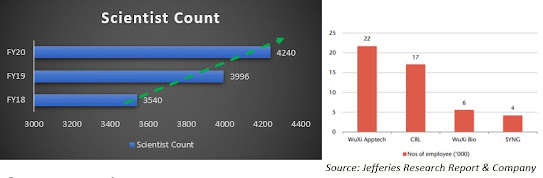
have been availing services for the last five years. It has a pool of 4240
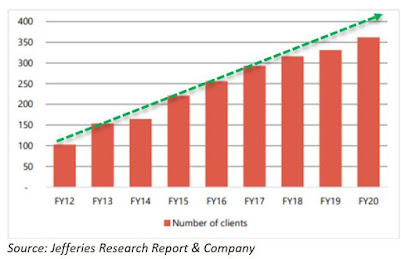
scientists. The client base has grown from 256 to 362 over FY16-20.
3. Segmental
Growth Year on Year from past 3 years
CRAMS and R&D
growth, Biologics is also in line for growth traction sequentially Revenue
increased 13% YoY to INR17.5b (in-line) in FY20, mainly led by strong growth in
Biologics (+31% YoY, 34% of sales) and Small Molecules (+16% YoY, 31% of
sales). Research Services (30% of sales) grew 11% YoY to INR5.2b. However,
Branded Formulations (9% of sales) de-grew by 26% YoY.
4. Rapid Increase in Revenue - Trend over the Years
Source: Company
Growth driven by increase in sales from
existing clients and acquisition of new clients
Engage, expand and extend strategy to
extend client relationship over a longer period of time
Growth in total number of clients
Increase in average revenue from largest
clients
Increase in number of services offered
to clients
5. Increase in number of Clients
6. Increase in number of Number of Asset
On a US$550 million program that
was spread over multiple years, the last year of which is FY21, it’s expected
to be fully invested with all US$550 million by the end of this year. During
the last 12 months, the company added US$108 million of that capex. With this
capital infusion, company’s fixed assets currently stand at US$425 million.
This includes an asset under construction of US$70 million.
Company is very much on track to
have a total asset base of US$550 million by the end of the FY21. Capex in FY20 stood at 108mn USD,
of which
• 43mn USD in API
• 28mn USD in Discovery
• 12mn in Biologics
• 25mn USD in others
• Total gross block stands at
451mn USD including 33mn USD of CWIP
• It commissioned a new research
facility (152K sq. ft.) supporting biology, QC microbiology and other research
domains.
• It is currently working at 70%
utilization and expects to hit 100% by end of May.
• It expects to achieve at least
1x asset turnover with revenue build-up by second
year of asset
• Revenue build-up for
manufacturing will take longer
8. Mangalore Facility
In FY 20
the company capitalized their Mangalore API plant which is reflected in the
number.US$100 million from the past was the total money company estimated for
the fully expanded capitalized plan. The US$75 million corresponds to the
current level of execution that company has targeted by FY21. But the company
plans in a later stage to expand it further. Total Capex
in the plant is USD 75mn and will be depreciated over 18yrs.
•
Construction is now completed
• It is in
validation phase
• It
expects to commence GMP commercial operations towards end FY21
9. Scientist Count
Scientist strength addition, the company has
typically added between 450 to 500 people on an annual basis over the last five
years. Now in FY20, the company added about 240 to 250 people. Syngene
still has large room to grow in terms of scientists.
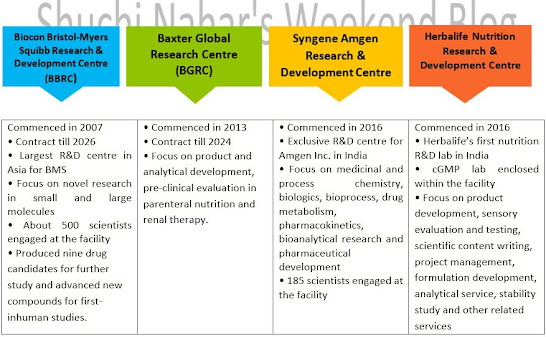
10. R&D Centres – Growth Engines
To maintain the structural balance and
improve profitability, they are inclined to outsource a substantial part of the
R&D work. Similarly, the innovative/virtual companies that are extensively
working on new products and which may not have the required capital/manpower
also tend to outsource a substantial part of their R&D. Track record
in acceptance of Recent Approvals
●
Now one of Syngene’s
strengths is their strong track record of regulatory compliance and over the
course of the last year, they have cleared audits not only by their clients, but also by regulators from across
the globe and that includes the US FDA, the European EMEA, Japanese PMDA and
others.
●
Collaborations and
partnerships to deliver numerous clinical candidates. Delivery history for
integrated CMC programs towards FIH and beyond. Company has recently been
approved for selling oral formulations in Russian markets.
●
It successfully cleared USFDA inspection
of the small molecule bioanalytical laboratory within the clinical development
service line with no observations. The company commissioned the first phase of
a new research facility in Bengaluru that will house discovery biology, QC
microbiology and other research capabilities.
●
Syngene undertaking manufacturing of
innovator drug APIs. It increases its revenue base, and also helps it offer
bundled and diversified services to its customers.
●
The company remains aggressive on the
capex front (~US$463 million already spent & another ~US$87 million
earmarked by FY21), attributable to order book visibility.
●
With elite client additions like
Amgen, Zoetis, Herbalife, GSK, etc, and multiple year extension of BMS and
Baxter contracts, the company remains well poised to capture opportunities in
the global CRO space.
●
The management has guided for
double-digit revenue growth on the back of continuous client additions, an
extension of existing contracts, increasing manufacturing and biological
contributions besides currency tailwinds.
Source: Syngene Annual Report & Investor Presentation
Jefferies Research Report
Axis Research Report
Disclaimer: The information provided on Shuchi Nahar’s Weekend Blog is for educational purposes only. The articles may contain external links , references and compilation of various publicly available articles. Hence all the authors are given due credit for the same. All copyrights and trademarks of images belong to their respective owners and are used for Fair Educational Purpose only.









Nice article suchi..
ReplyDeleteThank you Madam
ReplyDeleteSuperb Coverage..please keep up the good work
ReplyDeleteThank you for all the information, you rock :-)
ReplyDeleteThat is really an informative post. Genscriptprobio offers a one-stop service for antibody discovery and PK analysis. To know more about our Anti-idiotype Antibody services, contact us now.
ReplyDeletePlease mention the negatives also
ReplyDeleteI read your blog. A Contract Research Organization or Clinical Research Organization (CRO) is an assistance association that offers help to the drug and biotechnology businesses as reevaluated drug research administrations. The provided information is very useful for contract research organization in India. Keep continuing to post further.
ReplyDeleteI read your blog. Contract Research Organizations reduce the expense of innovative work to assist organizations and establishments with addressing the necessities of the advancing clinical device and pharma industry. The provided information is very useful for contract research organization. Keep continuing to post further.
ReplyDelete