Granules India Ltd - Large - Scale Vertically Integrated Company
Granules India Ltd. - Huge Scope of Growth Ahead
Twitter Handle: @shuchi_nahar
Company Overview
Founded
in 1991, Granules India Limited is a large-scale vertically integrated Company
manufacturing Active Pharmaceutical Ingredient (API), Pharmaceutical
Formulation Intermediate (PFI) and Finished Dosage (FD). The Company over the
years has created a strong presence in ‘the first line of defence’ products
such as Paracetamol, Ibuprofen, Metformin, Methocarbamol, and Guaifenesin.
Till
date, Granules has filed 45 ANDAs, received approval for 29 products (12 in
FY20) and launched five in the US. EBITDA margins have seen improving from
better product mix, operating efficiencies and higher capacity utilisations.
Also, the margins are expected to further expand with the launch of own label
products in the US, a new facility commenced at Vizag and using its own
Metformin with new capacity commissioned.
In FY20
it received approval for 12 ANDAs. It has a further 16 ANDAs (45 cumulative
filings) pending approval with the US FDA. The company plans to file 20-25 ANDA
filings from India and Virginia's facilities put together over the next three
years.
Company
has its dominance in key APIs such as paracetamol, ibuprofen and metformin are
likely to help Granules emerge as a prime beneficiary since the Covid-19
outbreak has led to a surge in demand for such drugs. Granules is likely to
maintain strong traction for the next couple of years, driven by formulations
and the healthy contribution from the new capacities.
Revenue & EBITDA trend over the years 2008 – 2020
Product Revenue Breakup - Recent 3 Years
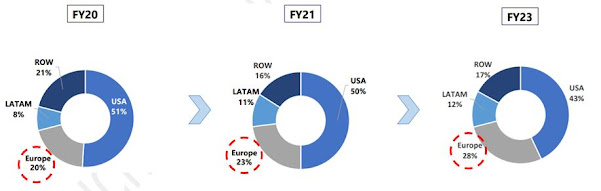
Geographic Revenue Breakup - Recent 3 Years
Under-Utilized
Capacity
Granules will focus on asset sweating as the newly
installed capacity is under-utilized. Key molecule capacity is underutilized
and waiting for regulatory approvals. Currently, Paracetamol capacity
utilization is at ~60- 65%, Metformin ~15% and Guaifenesin is undergoing
validation process.
Carlyle
Group in advanced stages to acquire Granules India for $1 billion:-
·
Granules India,
which has been on the block for a while may have found a buyer in large private
equity firm Carlyle Group, which has been active on pharma investments.
·
Granules
India has been exploring options to sell stake due to favourable valuations and
succession issues.
·
Carlyle
is in advanced stages to acquire Granules India for around $1 billion.
·
Carlyle
is expected to buy promoter shares which will trigger an open offer to acquire
additional stake from others. Carlyle recently acquired Sequent Scientific and
bought a minority stake in Piramal Pharma.
US Generics US
Generics - Opportunity from Core and Non-core products expansion:
In FY20 it received approval for 12 ANDAs. It has a further 16 ANDAs (45 cumulative filings) pending approval with the US FDA. The company plans to file 20-25 ANDA filings from India and Virginia's facilities put together over the next three years.
·
Received 2 awards from US government for products
made in GPI.
·
24 Rx ANDAs filed and 16 ANDAs approved currently. 8
products to be approved. 11 products under development between GPI and GIL (Rx)
·
Granules India Ltd US Generics: Granules
Pharmaceuticals Inc. (GPI)
·
Expect 3 launches in FY21, with an addressable
market size of about $1 Bn based on IQVIA (brand and generic). GPI
revenue in FY20 is at INR 2,960 Mn.
·
Core molecules include Paracetamol, Ibuprofen and
Metformin. Core molecules will keep increasing in absolute terms on an account
of expanding it to new geographies.
·
Gained traction with a first to generic product -
Methergine in June 2018. Set up a front end sales and marketing division and
launched 11 products under the GPI label: “Control your own destiny”.
·
Granules India expanding the base business by
entering into new geographies (Europe, Canada, South Africa).
·
The last paracetamol manufacturing plant in Europe
was closed in 2008, while India & China produces nearly 60% of the world’s
paracetamol.
·
Higher contribution from FD continues to grew in
absolute terms from INR 3,515 Mn to INR 4,301 Mn, up 22% YoY.
· Filing new ANDAs (7-8 ANDAs per year. 2-4 dossiers
per year and value added DMFs/CEPs/EDMFs).
·
Focus on developing controlled substances and
niche/differentiated modified and extended-release products in varied dosage
forms.
·
Average launches 7-8 ANDAs/Dossiers per year. Focus
on operational efficiencies and process innovation through R&D.
Disclaimer: The information provided on Shuchi Nahar’s Weekend Blog is for educational purposes only. The articles may contain external links , references and compilation of various publicly available articles. Hence all the authors are given due credit for the same. All copyrights and trademarks of images belong to their respective owners and are used for Fair Educational Purpose only.
Twitter Handle: @shuchi_nahar













Comments
Post a Comment