Jubilant Life Sciences-Unseen Opportunities Ahead

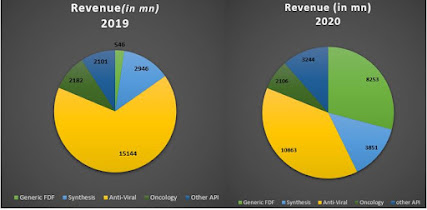
Jubilant Life Sciences-Unseen Opportunities Ahead (COVID-19 V accine manufacturing, Demerger of business inline) Twitter Handle: @shuchi_nahar Company Overview Incorporated in 1978, Jubilant Life Sciences (JLS; formerly Jubilant Organosys), is a mid-sized integrated chemical turned pharmaceuticals player. It started as a full-fledged chemical a company by entering the vinyl acetate monomer (VAM) business in 1983. Broadly, the company operates through two business segments - pharmaceuticals (62% of the turnover) and life science ingredients (35% of turnover). The pharmaceuticals segment consists of sub-segments like 1) generics 2) specialty pharma - radio pharma and allergy therapy products and 3) CDMO - contract manufacturing (CMO) of sterile injectables and API. In November 2020, Jubilant Life entered into a strategic partnership with SOFIE Biosciences, Inc. (SOFIE), US. Under the terms of the agreement, Jubilant will acquire 25% of equity holding for a cash consideration of