Hikal – An Unique CDMO Opportunities for Pharmaceutical, Animal Healthcare & Crop Protection

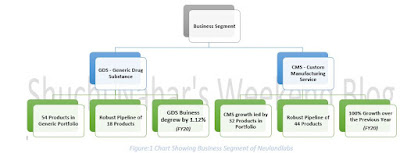
Hikal – Company Overview Shuchi.P.Nahar Twitter: @shuchi_nahar Link to my previous blog for detail explanation on What are CDMO and their wide scope: 1 . https://myweekendspot.blogspot.com /2020/07/ what-is-so-attractive-about-cdmos-in.html 2 . https://myweekendspot.blogspot.com /2020/07/a-masterclass-on-global-custom.html 1. Company Overview Established in 1988, Hikal is predominantly a B2B player that provides intermediates and active ingredients to global pharmaceutical, animal health, crop protection and specialty chemical companies. Hikal today is one of the leading global suppliers to the Life Sciences industry. Company is having six sites providing a multitude of services across the value chain. Figure:1 Business Snapshot The pharma business is currently divided almost equally between generic active pharma ingredients (APIs) and contract development and manufacturing organisation (CDMO) businesses. Leading Sustainable Technology driven company serving the Crop Protecti